Publication
Modelling the effect of vascular status on tumour evolution and outcome after thermal therapy
Jesús J. Bosque, Gabriel F. Calvo, María Cruz Navarro
Applied Mathematical Modelling 110 (2022) 207–240
MOLAB authors
Abstract
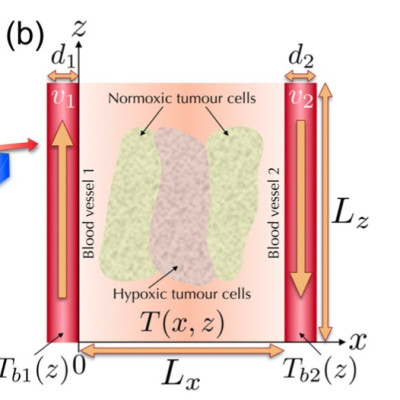
Microscale oxygenation plays a prominent role in tumour progression. Spatiotemporal vari- ability of oxygen distribution in the tumour microenvironment contributes to cellular het- erogeneity and to the emergence of normoxic and hypoxic populations. Local levels of oxy- gen strongly affect the response of tumours to the administration of different therapeutic modalities and, more generally, to the phenomenon of resistance to treatments. Several interventions have been proposed to improve tumour oxygenation, being the elevation of the local temperature (hyperthermia) an important one. While other factors such as the metabolic activity have to be considered, the proficiency of the tumour vascular system is a key factor both for the tissue oxygenation and for its temperature maps. Consequently, the interplay of these factors has attracted considerable attention from the mathematical modelling perspective. Here we put forward a transport-based system of partial differen- tial equations aimed at describing the dynamics of healthy and tumour cell subpopula- tions at the microscale in a region placed between two blood vessels. By using this model with diverse flow conditions, we analyse the oxygen and temperature profiles that arise in different scenarios of vascular status, both during free progression and under thermal therapy. We find that low oxygen levels are associated to elevations of temperature in loca- tions preferentially populated by hypoxic cells, and hyperthermia-induced cell death, being strongly dependent on blood flow, would only appear under highly disrupted conditions of the local vasculature. This results in a noticeable effect of heat on hypoxic cells. Addition- ally, when pronounced cell death occurs, it is followed by a significant increase in the oxygen levels. Our results provide quantitative insight to the physiological and biological processes taking place at sub-voxel sizes, currently not accessible to standard functional imaging due to spatial resolution limitations.