Publication
Pretreatment inflammatory indices predict Bevacizumab response in recurrent Glioma
A. Martínez-González, R. Cabrera, M. Lloret, P.C. Lara
Cancer Drug Resist 3, 623-635 (2020)
MOLAB authors
Abstract
Aim: It remains unclear what the best therapeutic option for recurrent glioma patients after Stupp treatment is. Bevacizumab (BVZ) is commonly administered in progression, but it appears that only some patients benefit. It would be useful to find biomarkers that determine beforehand who these patients are.
Methods: The protocol included 31 high-risk progressing glioma patients after Stupp treatment who received BVZ 5-10 mg/kg every 14 days and temozolomide (3-19 cycles, 150-200 mg five days each 28-day cycle) during a mean of eight cycles of BVZ or until tumor progression or unacceptable toxicity. We analyzed the clinical outcome values of inflammatory indices measured before BVZ administration.
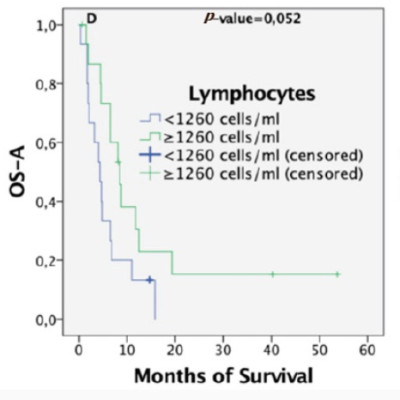
Results: Lymphocyte level before BVZ administration was the best independent predictor of overall survival (HR = 0.34; 95%CI: 0.145-0.81; P = 0.015). The area under the receiver operating characteristic (ROC) curve was 0.823, with 1.645 being the optimal cut-off value, and 0.80 and 0.85 the sensitivity and specificity values, respectively. Responder and non-responder survival curves were also significantly different, considering the first and second tertiles as cut-off points. The number of BVZ cycles was not related to lymphopenia. Pretreatment neutrophil, platelet levels, platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR) did not have independent predictive value. Inflammatory variables were not correlated with each other. However, patients with high NLR and PLR simultaneously (double positive PLR-NLR) showed a worse clinical outcome than the rest (P = 0.043).
Conclusion: Pretreatment lymphocyte levels and double positive PLR-NLR could be used as non-invasive hematological prognostic markers for recurrent gliomas treated with bevacizumab. A close relationship emerged between inflammation and angiogenesis.