Publication
Glucose-lactate metabolic cooperation in glioma tumours: insights from a spatial mathematical model and implications for targeted therapy
J.B. McGillen, C.J. Kelly, A. Martínez-González, N.K. Martin, E.A. Gaffney, P.K. Maini, V.M. Pérez-García
Journal of Theoretical Biology 361, 190-203 (2014)
MOLAB authors
Abstract
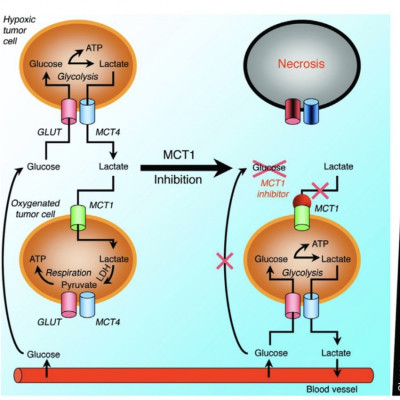
A recent hypothesis has proposed that glucose-lactate cooperation occurs between adjacent hypoxic and oxygenated regions of developing tumours, and is a promising target for a two-stage targeted treatment strategy. This hypothesis is controversial, however, and in vivo experimental evidence remains inconclusive. Here we develop a minimal spatial mathematical model of glucose-lactate metabolism to determine whether metabolic cooperation is plausible in human glioma tumours and assess the potential impact of inhibiting this cooperation. We find that, although glucose-lactate cooperation is permitted in a large region of the fundamental parameter space, it does not straightforwardly prevent glucose starvation in the tumour interior, and stronger cooperation is not advantageous for tumour growth over weaker cooperation. Moreover, strongly cooperative behaviour is observed only under certain conditions on the model parameters; and these, we demonstrate experimentally, are unlikely to be satisfied in U87 brain tumours. These results suggest that physiologically realistic glioma tumours are likely to exhibit weak metabolic cooperation at most. Finally, we show that the aforementioned targeted treatment strategy is not effective under our modelling framework.